The Purse Group at SDSU is a diverse team of graduate and undergraduate researchers using the tools of synthetic and physical organic chemistry to create new nucleoside analogues for applications in biophysical studies and chemical biology and to create and study self-assembling molecular systems. We seek to advance chemical knowledge for rational molecular design while providing useful tools to illuminate, manipulate, and study biology.
Research Highlights
Purse Lab paper: Chem Sci 2025, 16, 4866–4875.
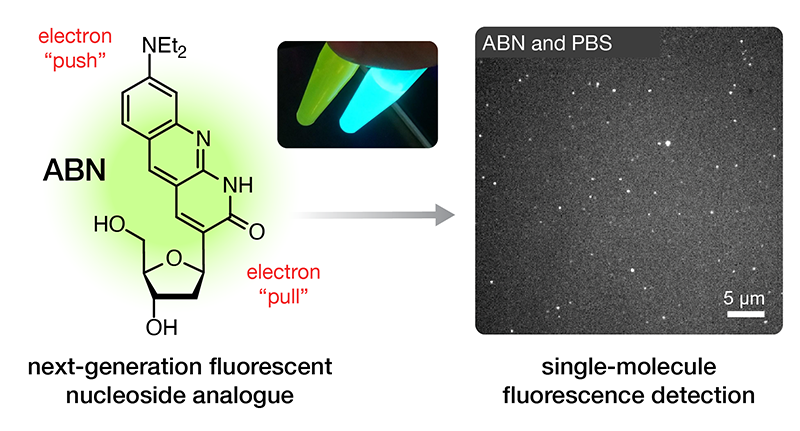
Single-molecule detection of oligonucleotides using the fluorescent nucleobase analogue ABN
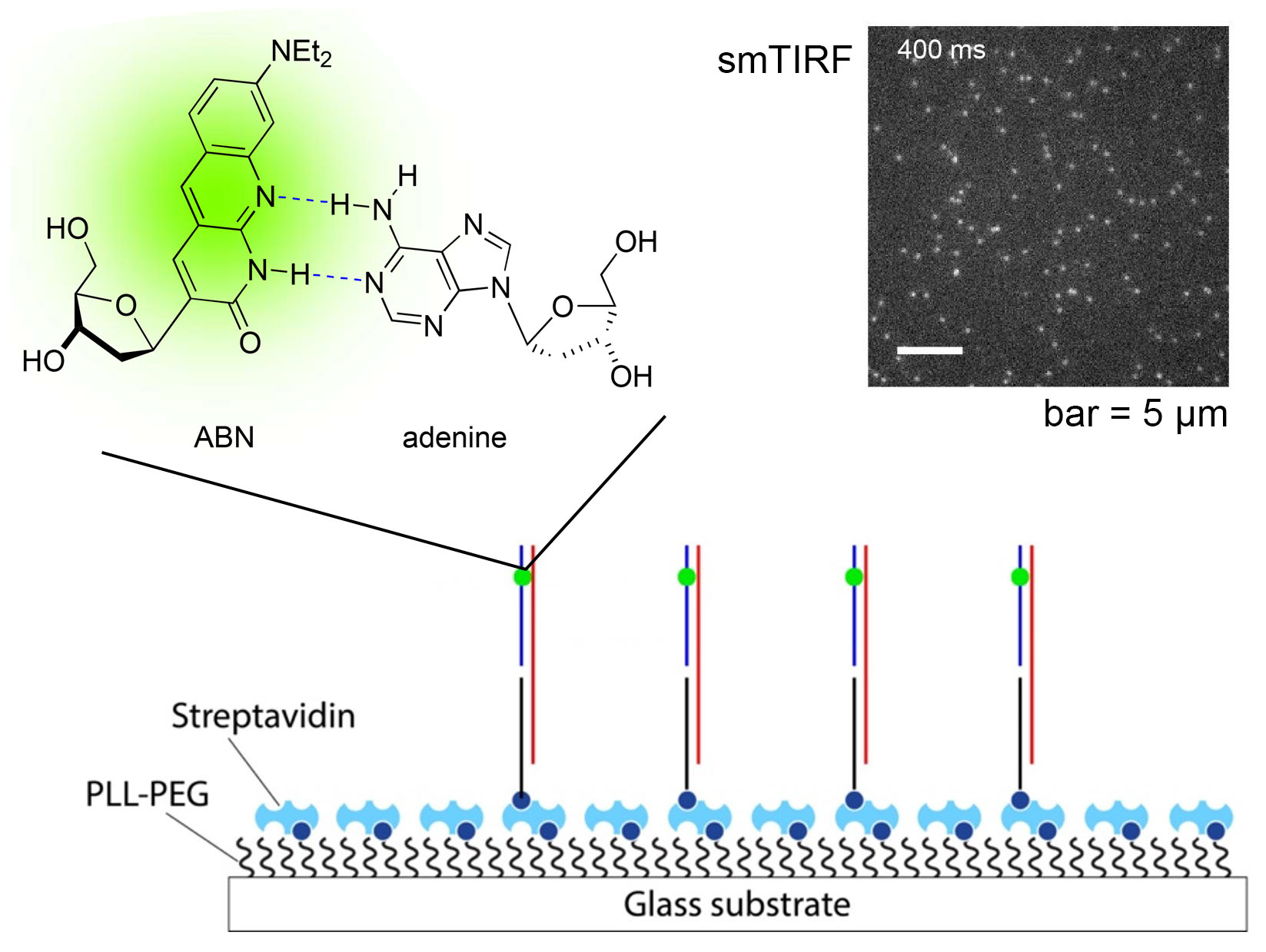
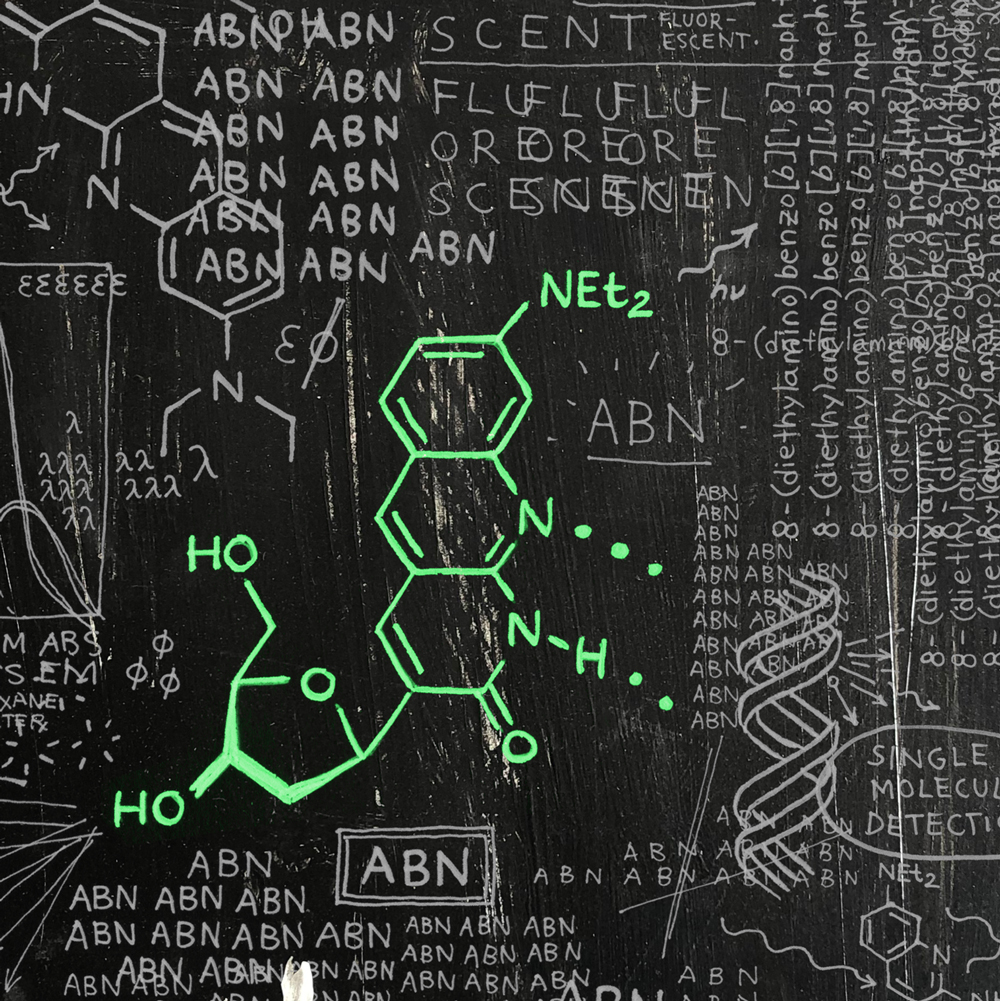
We’re thrilled to announce the publication of our latest paper, this one demonstrating that our fluorescent nucleobase analogue ABN provides very favorable spectral characteristics and quantum yield when based paired and stacked in duplex DNA oligonucleotides. By favoring a high quantum yield thymine-like tautomer through base pairing, surface-immobilized ABN-containing DNA duplexes are readily observed as bright spots using single-molecule fluorescence microscopy, exhibiting well-defined single-exponential bleaching kinetics. These results demonstrate that ABN is unique among FBAs in enabling single-molecule fluorescence studies of oligonucleotides using a standard microscopy setup.
Purse Lab paper: Bioconj. Chem. 2023, 34, 1601–1071.
Sequence-Specific Fluorescence Turn-On Sensing of RNA by DNA Probes Incorporating the Tricyclic Cytidine Analogue DEAtC
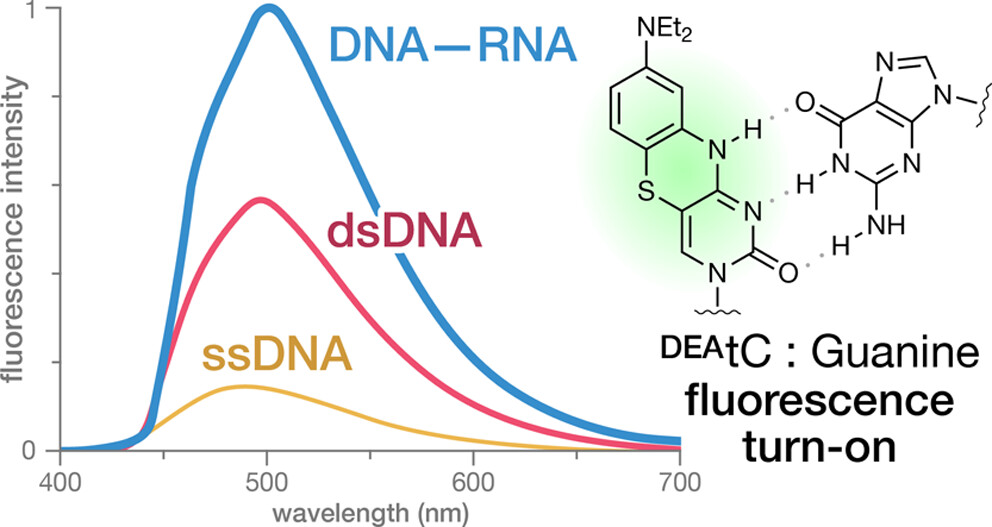
Purse Lab graduate students Ben Turner, Dana Rosansky, and Jesús Ceja, undergraduates Julian Cizmic and Grace Kim, and our collaborators in Tammy Dwyer’s lab at USD have published an exciting new study showing that synthetic DNA oligonucleotide probes containing our fluorescent nucleobase analogue DEAtC exhibit an average 8-fold increase in fluorescence intensity when hybridized to matched RNA with DEAtC base paired with G and little fluorescence turn-on when DEAtC is base paired with A. These sequence-specific fluorescence turn-on probes can immediately differentiate between match and mismatched sequences in hybrid duplexes.
Purse Lab paper: J Am Chem Soc 2022, 144, 14647–14656.
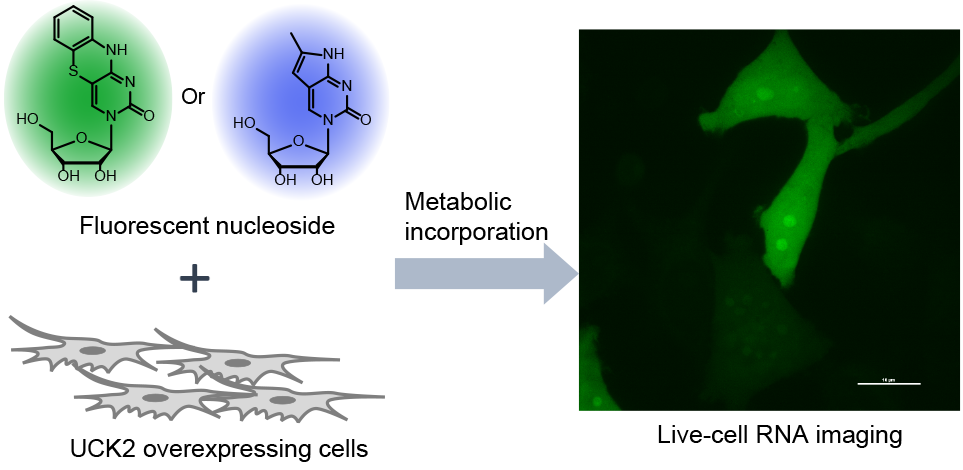
Ani Shalamberidze, a Ph.D. student member of our team, has published a JACS paper in collaboration with the Kleiner Lab at Princeton shows that a combination of judiciously selected fluorescent nucleoside analogues with genetic upregulation of nucleoside kinase activity enables metabolic labeling and live-cell imaging of RNA biology. The method has already been used to discover previously unknown cytoplasmic RNA granules generated during oxidative stress. Great ready for more discoveries in RNA biology!
Purse Lab paper: Chem Sci 2021, 12, 2623-2628.
Purse Lab Ph.D. student George Samaan has develop the first fluorescent nucleobase analogue that performs robustly in single-molecule fluorescence measurements. Get ready for exciting new advances in single-molecule biophysical studies on nucleic acids!
Purse Lab paper: ChemPlusChem 2020, 85, 855–865.
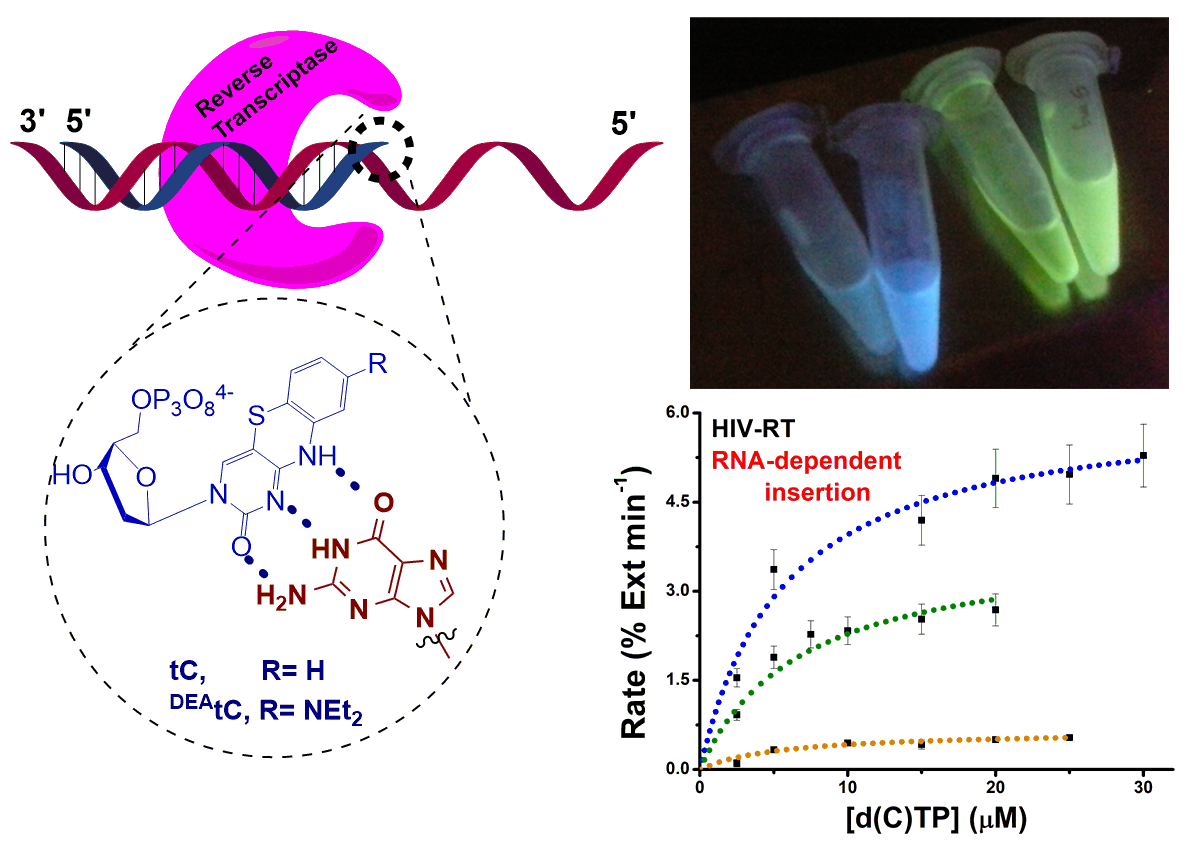
Purse Lab Ph.D. student Ben Turner has published a new paper showing that fluorescent tricyclic cytidine analogues are substrates for the reverse transcriptases (RTs) of three retroviruses: avian myeloblastosis virus (AMV), Moloney murine leukemia virus (M‐MLV), and human immunodeficiency virus 1 (HIV‐1). These RTs use the fluorescent substrate analogues to synthesize a fluorescently labeled double-stranded DNA from an RNA template, with possible future applications in studying retroviral replication. Read more here!
Purse Lab paper: Bioorg. Med. Chem. Lett. 2020, 30, 126818.
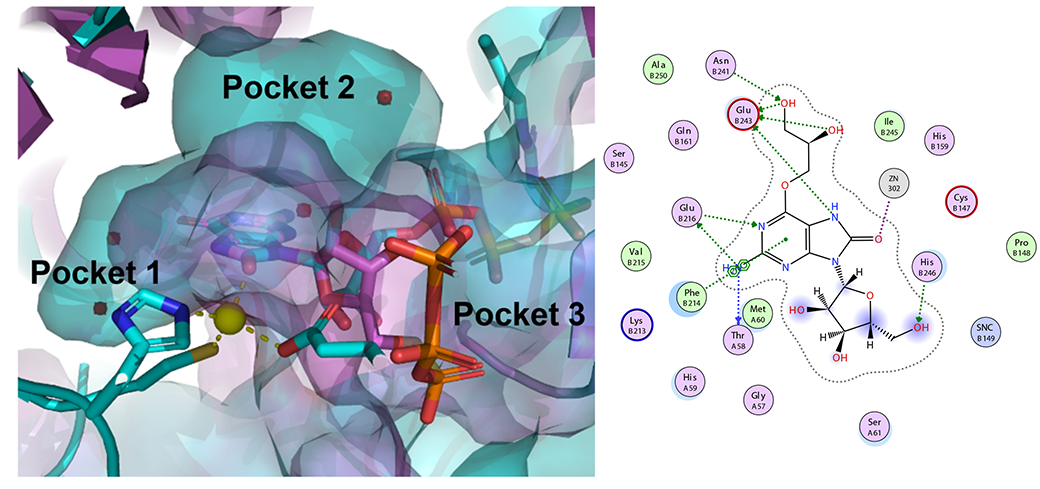
Ph.D. student George Samaan of the Purse Lab has designed, synthesized, and tested new 8-oxoguanosine analogues to inhibit GTP cyclohydrolase IB, an enzyme essential for the growth and survival of some pathogenic bacteria including N. gonorrhoeae. George’s strategy, which includes further designs that we will publish in the future, presents an opportunity to create a new class of antibiotics against resistant pathogens. Read all about it here!
Purse Lab paper: Org. Chem. Front. 2019, 6, 1361–1366.
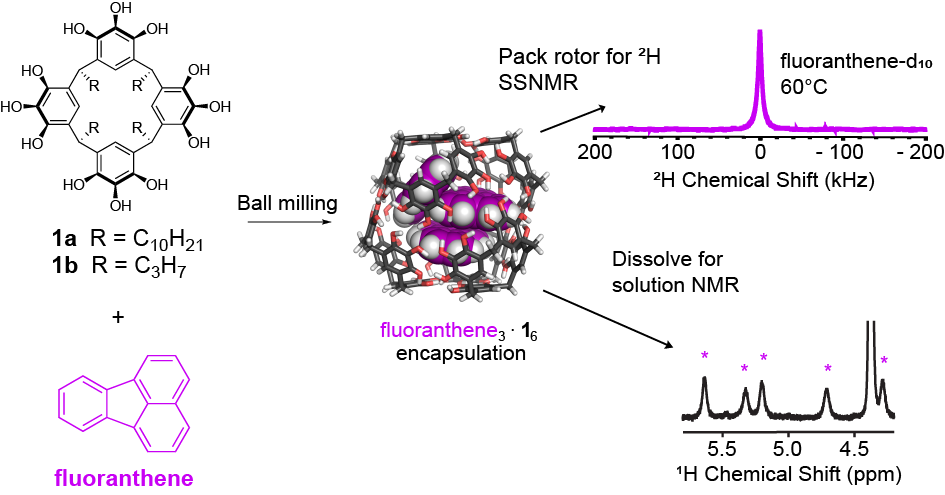
Purse Lab students led by undergraduate researcher Irazema Islas, along with collaborators in the Holland Lab at SDSU, have published a paper showing that self-assembled molecular capsules prepared by ball milling offer liquid state-like interiors with the tiny volume of 1 nm^3! Click here to read about the team’s insights into this remarkable nanoscale chemical environment.
Purse Lab paper: J. Am. Chem. Soc. 2017, 139, 1372–1375.
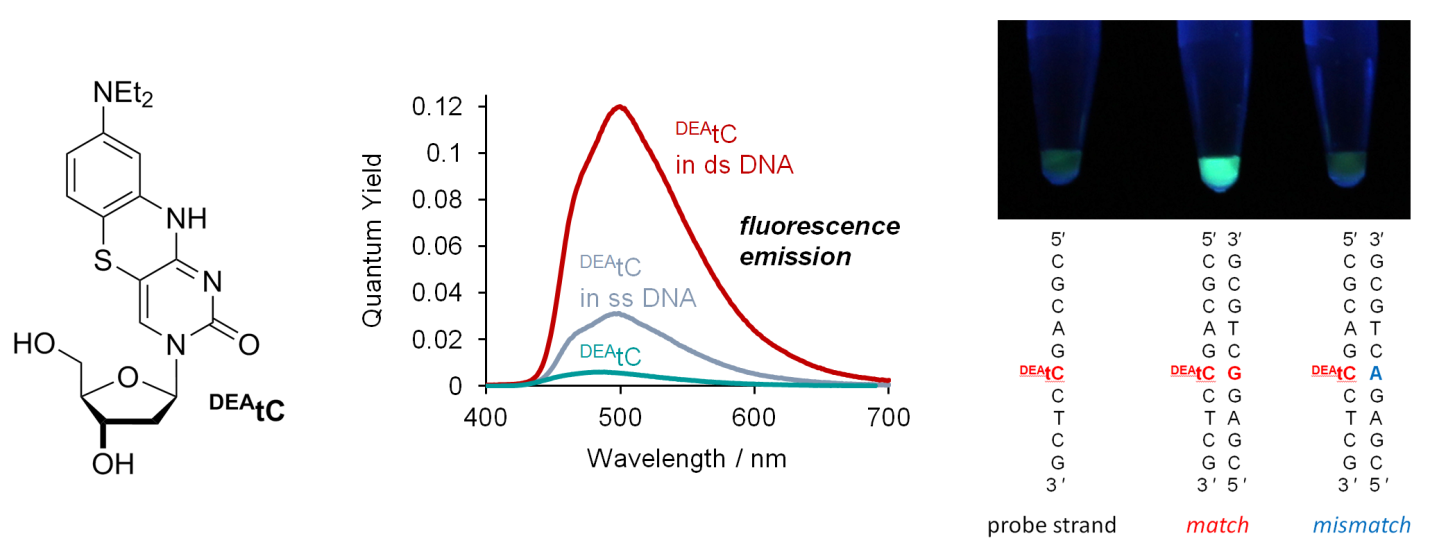
The Purse Lab has invented a new cytidine analogue that becomes vastly more fluorescent upon incorporation into double-stranded DNA. Remarkably, the fluorescence turn-on response is specific to correct base pairing with guanosine, allowing visual discrimination of matched sequences, as shown in the photo below (left to right: single stranded probe, matched duplex, mismatched duplex). This fluorescence turn-on response promises new applications in biophysics. Read the details here!