Nucleosides are the building blocks of DNA and RNA, the biomolecules that store the genetic code and play critical roles in regulating cellular metabolism. They are used as the energy currency of cells (ATP is a nucleoside triphosphate), as precursors of metabolic cofactors (FAD and NADPH), for controlling chemistry in cells (catalytic RNA in ribosomes), and as chemical transporters (tRNA). Their importance cannot be overstated! Chemists have an interest in modifying the structures of nucleosides to make new molecular probes (e.g. fluorescent molecules that can be used to track and study nucleic acids) and to make drugs. Many important medicines are synthetic derivatives of nucleosides that “trick” infected or malignant cells into using the “unnatural” nucleoside by mistake during replication. This mechanism is how azidothymidine (AZT) attacks the HIV virus, gemcitabine kills breast cancer cells, and sofosbuvir can be used to cure hepatitis C. Back to the former application, fluorescent nucleoside analogues are essential tools for studying the mechanisms of copying, maintenance, and regulation of expression of the genetic code. They are also vital to modern, next-generation DNA and RNA sequencing methods.
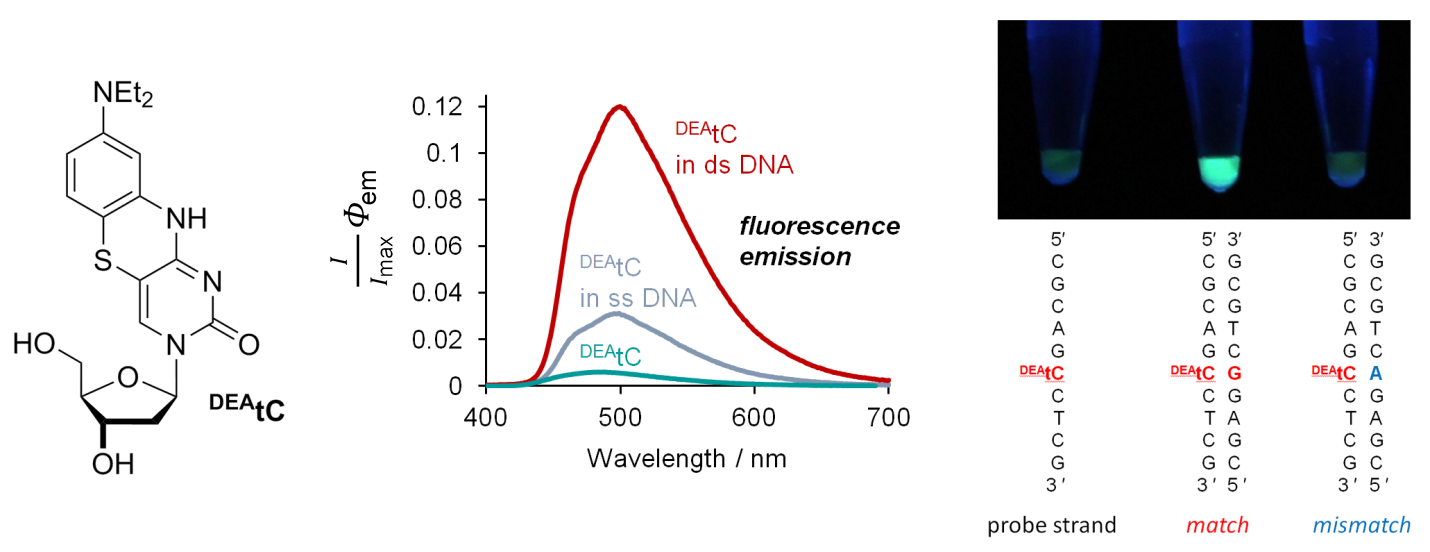
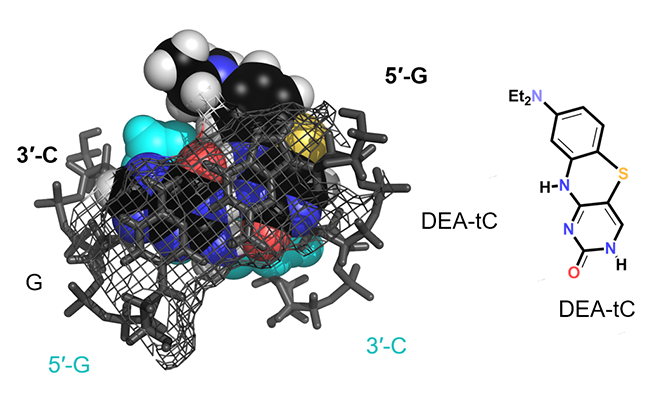
Our lab is interested in developing new nucleoside analogues both for unmet needs as molecular probes, in chemical biology, and in medicinal chemistry. We are currently working on synthetic elaboration of natural nucleosides to impart useful fluorescent properties, while minimizing the biochemical impact of the required structural changes. Recently we discovered a way to modify cytidine—the C of the genetic code—to create a fluorescence turn-on property. This new compound, which we call DEAtC, is able to report on the formation of matched double-stranded DNA and RNA by becoming fluorescent (see image above and read more here).
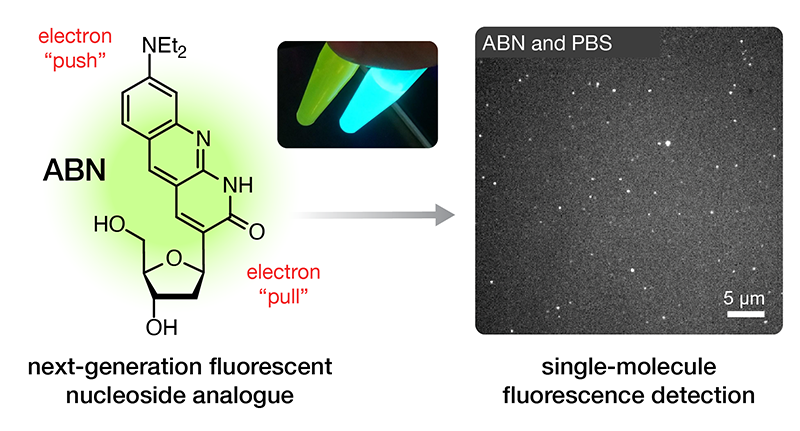
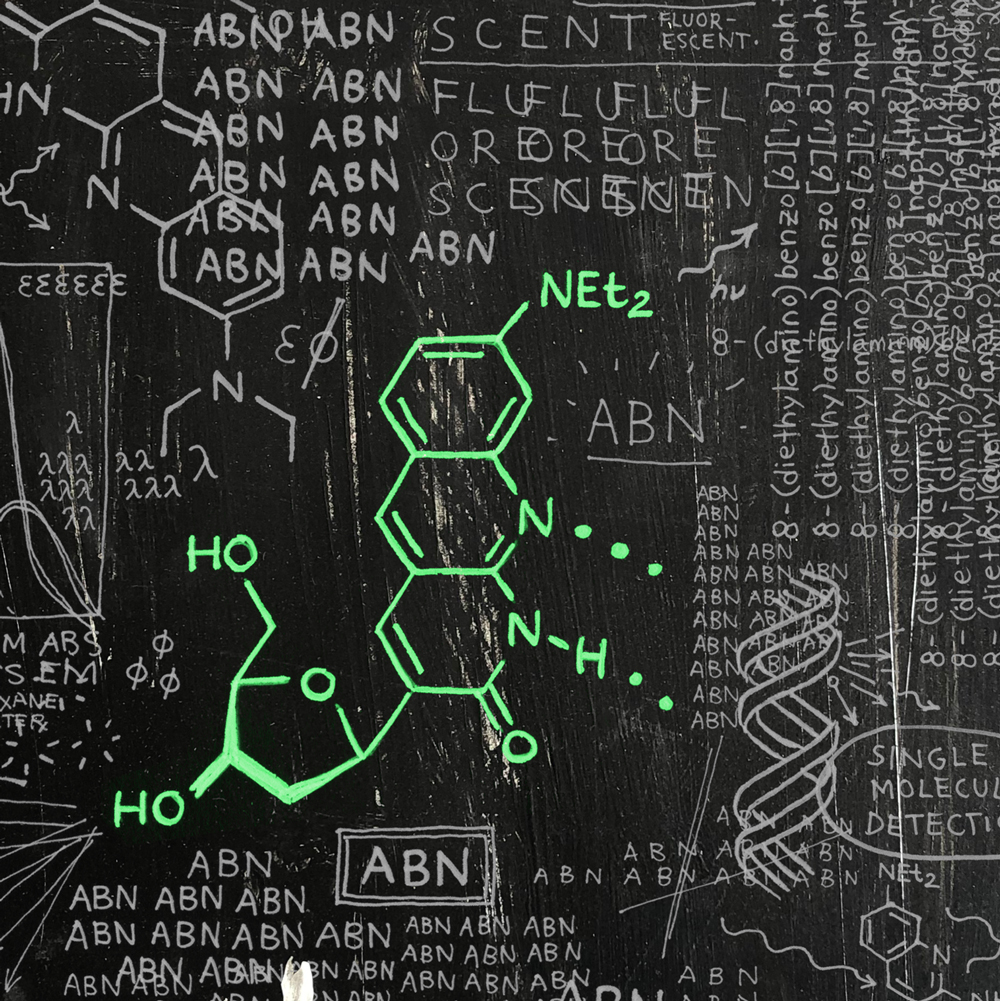
We have also worked to develop fluorescent modifications of pyrimidine nucleobases that are sufficiently bright as to enable single-molecule fluorescence measurements—studying one molecule at a time. Our new fluorescent nucleoside analogue ABN is the first surrogate for a natural nucleobase that is a sufficiently bright fluorophore to allow robust detection at the single-molecule level while retaining the capacity to engage in Watson–Crick–Franklin base pairing (see image above and read more here).
Currently we are working on further improvements to this and other fluorescent properties and are carrying out studies of structure and photophysics to inform the next rounds of fluorescent probe design. We are also working on applications of this new method for rapid detection and discrimination of genetic information.
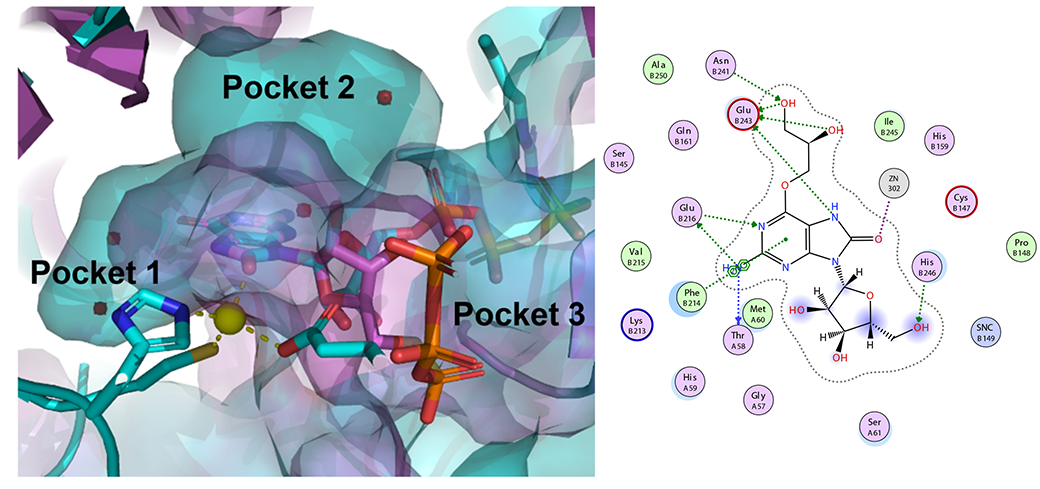
Other members of the Purse Lab are working on nucleoside analogues as prospective new medicines. We are using synthetic chemistry to re-design the natural substrates for bacterial and viral enzymes to selectively poison the metabolism of pathogens. Current efforts are focused on the Zika virus and resistant strains of N. gonorrhoeae (see image above and read more here).
For more information, see our Publications.