The field of supramolecular chemistry is concerned with the study of how and why molecules can self-assemble into higher-order structures with emergent properties. Throughout the history of the field, most studies and applications have used assemblies at equilibrium, where there is dynamic exchange of components between assemblies. Our group is interested in self-assembled supramolecules with high kinetic stability; that is, those whose components exchange slowly or not at all. Such assemblies offer the potential for applications in changing environments where component exchange cannot be tolerated, such as in living cells.
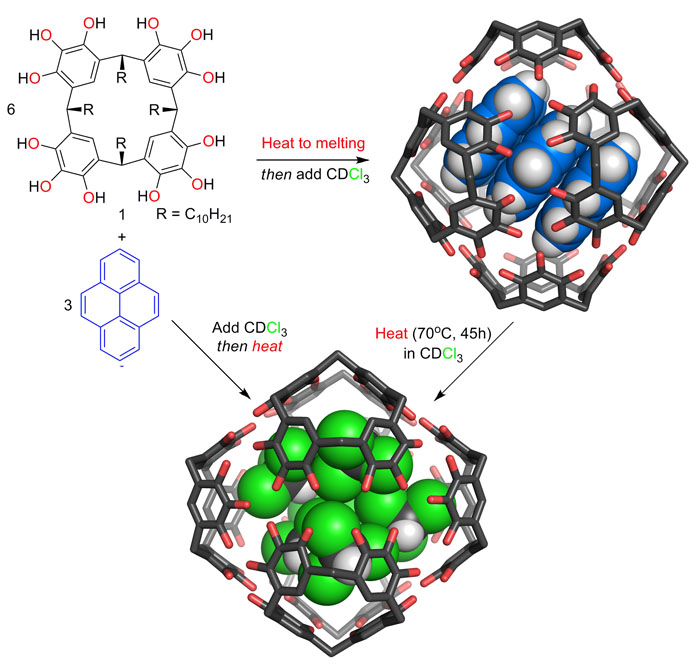
Some of the earliest research in the Purse Lab led us to identify the most kinetically stable known molecular capsules that are held together by hydrogen bonds. These capsules will indefinitely trap their occupants at ambient temperatures even as thermodynamics would prefer the release of the capsules’ guests. Heat is required to break the capsules apart and release their occupants. Noting the special stability of these capsules, we have turned our attention towards embedding these capsules in materials that can channel mechanical forces to control capsule opening and closing. The long-term goal of our work in this area is to create new smart materials that can release or take up specific small molecules in response to mechanical forces.
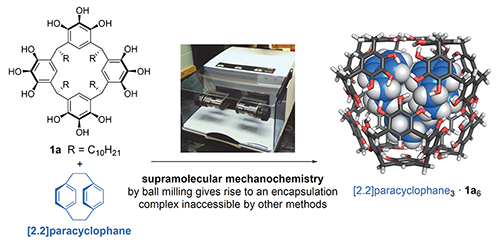
Recently, we have shown that mechanical forces can be used to drive the assembly of multi-component molecular capsules with guest entrapment entirely in the solid state. By using a ball mill to grind powdered mixtures of capsule components and guest molecules, we demonstrated that this solid-state synthesis method can be used to produce novel encapsulation complexes that are not stable in solution and that cannot be prepared using any other known method. Our results suggest that solid-state synthesis has an untapped potential to expand the scope of complex materials that can be made only by molecular self-assembly.